The Liver-Brain-Sugar Axis
The liver is a core regulator of sugar. In addition to handling cholesterol and fats which I won’t discuss today, it stores sugar and provides backup power to the brain during fasting states. It’s also the detoxification center of the body and the primary site of active thyroid conversion. With so many functions for the body and so many assaults from the environment, prioritizing liver health is key for high energy.
TLDR:
The liver plays a central role in glucose metabolism, and its relationship with sugar (glucose) is crucial for maintaining energy homeostasis.
Liver glycogen (storage glucose), is the fuel reservoir for the brain during sleep and fasted states.
The liver is the primary site of T4 to T3 conversion.
Impaired liver function can significantly reduce serum T3 levels, cause hypothyroidism and reduce glycogen storage.
Sugar the preferred fuel of the brain. Not only does high metabolic rate in the neurons correlate with improved cognition, its correlated with improved mood and emotional regulation.
Sugar (with low fat) and choline are superfoods for the liver.
In the fed state, the liver predominantly oxidizes glucose through glycolysis and the tricarboxylic acid (TCA) cycle to generate ATP. During fasting, the liver shifts to fatty acid oxidation for energy to conserve glucose for other tissues, such as the brain and red blood cells.
After a meal, the liver takes up glucose from the bloodstream under the influence of insulin. Glucose not used for energy production is stored as glycogen through a process called glycogenesis. This stored glycogen can later be broken down during fasting to maintain blood glucose levels (glycogenolysis).
If energy needs are met and glycogen storage is full excess glucose is converted into fatty acids via lipogenesis and stored as triglycerides. This process is known as de novo lipogenesis (DNL). DNL also occurs when both fat and sugar intakes are high, especially when exceeding your total daily expenditure (TDEE).
Once glucose and glycogen are used up, as in a prolonged fasted state, the liver produces glucose through gluconeogenesis (discussed last week) to prevent hypoglycemia.
Insulin promotes glucose uptake into the cell, glycogenesis, and glycolysis while inhibiting gluconeogenesis and glycogenolysis. Glucagon has the opposite effect, stimulating gluconeogenesis (with cortisol) and glycogenolysis during fasting or low blood sugar states. In the fed state the liver stores and utilizes glucose to lower postprandial blood sugar levels. In the fasting state it produces and releases glucose to maintain energy supply for vital organs like the brain.
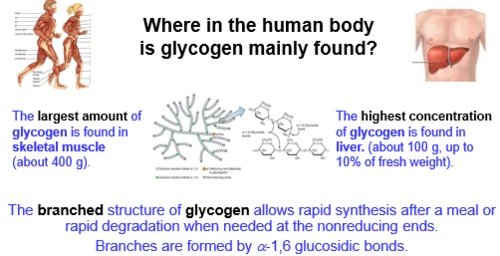
The largest depots of glycogen storage in the body are skeletal muscle (300-700g range) and the liver (0-160g range). Glycogen storage capacity in humans is approximately 15 g/kg body weight, but this can vary based on carbohydrate consumption and training status. Increasing glycogen storage improves exertion capacity and overall performance. It reduces risk of muscle damage and overtraining. And provides of a readily available energy source when blood glucose levels decrease maintaining stable blood glucose levels, especially for brain function.
Glycogen storage capacity is adaptable within certain limits, and dietary carbohydrate intake plays a significant role in this adaptation. Prolonged low-carb diets decrease glycogen capacity.
The body's ability to store glycogen can vary based on training status, with even short periods of detraining reducing muscle glycogen stores. Regular training coupled with a high-carbohydrate diet (50%+ of TDEE) leads to supercompensated muscle glycogen stores.
Muscle glycogen is for local use in the muscles that store them ie physical work, fitness, competition. Liver glycogen is largely a storage warehouse for the brain.
Adequate liver glycogen ensures a steady supply of glucose during fasting or physical activity, reducing the risk of hypoglycemia-related symptoms like fatigue, confusion, or fainting. Stable blood sugar levels are critical for optimal brain function, including memory, focus, and decision-making. Emotional regulation is highly dependent on sufficient sugar. A 50%+ carbohydrate diet (250-300g) supports a lower stress state emotionally, cognitively and physically.
During championship chess games, glycogen stores are rapidly depleted due to high neuronal function, forcing the body to rely on lipolysis and gluconeogenesis for energy. This metabolic shift is accompanied by increased stress hormone activity, which mobilizes stored fats and proteins to maintain energy supply.
This slower energy production process can impair mental performance over time, highlighting the importance of adequate glycogen stores for sustained cognitive activity.
Sleep is a fasting state, and the liver plays a crucial role in maintaining blood glucose levels through glycogenolysis. Glycogen from the liver is broken down to glucose and shuttled in the bloodstream for uptake by the brain. Without sufficient liver glycogen, hypoglycemia occurs and disrupts sleep by triggering stress hormones like cortisol to mobilize alternative energy sources.
REM sleep is a high brain activity state similar to wakefulness with both exhibiting strong beta wavelengths. In these states more glucose is needed. Since sleep trends toward more REM throughout the night with the highest amount close to waking, energizing this process with sufficient glucose is critical. If you’re predominately a fat-burner (and by association have low glycogen storage), the tendency is to wake up with shorter duration sleep and insufficient REM.
This process acts as a negative feedback in that sleep loss contributes to impaired glucose metabolism and increased risk of diabetes mellitus through increased free fatty acid burning.
Approximately 60% of T4 is converted to T3 in the liver, primarily through the action of deiodinase enzymes (Dio1). Elevated bilirubin and reduced albumin, markers of liver dysfunction, are correlated with poor T4-to-T3 conversion.
Chronically elevated stress hormones impair T4-to-T3 conversion by redirecting T4 toward reverse T3 (rT3), which slows metabolism. About 20% of T4 is converted to T3 by gut bacteria and this process depends on a healthy microbiome.
Adequate levels of selenium and zinc are required for the activity of deiodinase enzymes responsible for converting T4 to T3. Also, elevated estrogen increases thyroid-binding proteins, reducing free thyroid hormones available for cellular uptake and conversion in the liver.
Sufficient choline and glucose are magic for the liver. High PUFA fat diets, and choline deficiency are strongly linked to the development of fatty liver disease. Studies have shown that choline supplementation can eliminate fatty liver in patients receiving total parenteral nutrition (TPN). When choline was added to TPN solutions, liver fat disappeared and blood choline levels normalized.
In animal studies, choline deficiency exacerbates fatty liver induced by high-fat diets. Conversely, this suggests that adequate choline intake may help protect against diet-induced fatty liver. Choline is vital for fat metabolism in the liver and helps regulate homocysteine levels. It supports the production of phosphatidylcholine, which is necessary for the export of fat from the liver.
Human studies have found associations between low dietary choline intake and increased risk of NAFLD, particularly in normal-weight women. Egg yolks and beef liver are the highest food-based sources of choline. For individuals that have a history of high PUFA fat diets and/or fatty liver I recommend Mitolipin. It’s the only saturated fat source of choline in supplement form.
Metabolic dysregulation is associated with an increased risk of emotional dysregualtion. High levels of glucose and triglycerides, along with low levels of high-density lipoprotein, were linked to a higher future risk of these psychiatric disorders.
Patients with Exhaustion Disorder (ED) showed reduced functional activity in the prefrontal cortex compared to healthy individuals. This altered activity was observed during cognitive tasks, suggesting differences in information processing in the PFC.
Mental fatigue, which is often associated with negative emotions, is linked to reduced activity in the left lateral prefrontal cortex (LPFC).
Many people report that their mood and cognition is more stable while being in ketosis. That’s great. But I argue it isn’t an intrinsic property of ketones to stabilize mood. Being under-fed with carbohydrates (outside of ketosis) causes the emotional destabilization. People simply needed to eat more. Glucose oxidation is a high energy state creating the highest cognitive and emotional performance.
To your health,
Jonathan
This is for informational purposes only and should not replace professional medical advice. Consult with your physician or other health care professional if you have any concerns or questions about your health.