Your Stress Tolerance Threshold
The word stress was first introduced into the medical lexicon in 1936 by endocrinologist Hans Selye. He demonstrated that stressors, either physical or emotional, produce a consistent physiological response involving the hypothalamus, pituitary gland, and adrenal glands (HPA axis). He also proposed that chronic stress contributes to diseases like hypertension, ulcers, heart disease, and autoimmune disorders. Selye noticed a repeatable pattern in humans and animals he termed General Adaptation Syndrome (GAS).
Today I’ll discuss how your net stressors (costs) balance against your energy production (revenue) to determine your net Stress Tolerance Threshold (surplus). And why this needs to be considered in your personal context when choosing what to eat how to move.
TLDR:
GAS theory contains 3 stages: Alarm, Resistance and Exhaustion and your movement through these stages is determined by your stress tolerance.
Stress tolerance is a composite of your stressors and metabolic rate, composed of your mitochondrial and thyroid function.
The thyroid-stress hormone (adrenaline-cortisol) axis is the main determinant of metabolic rate and downstream mitochondrial energy output.
Hormesis, the idea that a little stress makes you more resilient, in the context of GAS is simply an energy expenditure with no valuable return.
Parental metabolic health shapes offspring metabolic rate through genetic (SNP-driven) and epigenetic mechanisms, creating multigenerational adaptive effects.
General Adaptation Syndrome (GAS) is a three-stage physiological response to stress: alarm, resistance, and exhaustion. The alarm stage is the initial "fight or flight" response when there’s a release of stress hormones like adrenaline, noradrenaline and cortisol causing increased heart rate and blood pressure, and heightened alertness. Typically, if the stressor disappears an individual returns to homeostasis.
However, if the stressor(s) persist, or are intense enough, one enters the Resistance stage - an adaptation phase where the body chronically elevates stress hormones and remains on high alert. It is here that symptoms can arise like irritability, poor concentration, and insomnia.
With continued persistance of stressors, one reaches the last stage, Exhaustion, where the body is depleted of resources and where illnesses develop. Here there’s a noticeable intolerance to stress, chronic fatigue, anxiety, and/or depression.
All of us have a different threshold of when we’re moved from Homeostasis to Alarm, Alarm to Resistance and Resistance to Exhaustion. This threshold is determined by cellular energy production (mitochondrial health), thyroid status, stress hormone status and the composite metabolic rate.
Mitochondrial dysfunction, which leads to reduced ATP availability, lowers the threshold for entering the Alarm stage and accelerates progression to Exhaustion. Stress hormones like adrenaline, noradrenaline and cortisol depress thyroid hormones, progesterone and androgens.
Thyroid hormones (T3/T4) regulate basal metabolic rate and mitochondrial efficiency. Impaired thyroid function reduces energy availability, making individuals more prone to stress-induced transitions between GAS stages. High metabolic rates improve resilience during the Resistance stage by maintaining cellular energy balance.
Your ability to stay in Homeostasis or return to Homeostasis after Alarm depends on mitochondrial health, T3 hormone production, and subsequent metabolic rate.
Here’s a basic equation to understand these concepts:
[Metabolic Rate] - [Net Stressors (physical and emotional)] = Your Stress Tolerance Threshold (movement through GAS stages)
Ray Peat integrated Hans Selye’s GAS framework into his bioenergetic model by emphasizing energy metabolism as the foundation for stress resilience. Peat is known for explaining this as follows “Stress consumes energy that would otherwise maintain structure. Without adequate energy, structure fails.”
Acute stress triggers cortisol and adrenaline release, which Peat linked to mitochondrial inefficiency and reliance on anaerobic glycolysis (lower ATP yield). Chronic stress depletes energy reserves (e.g., ATP, glycogen), forcing tissues to prioritize survival over repair. Peat tied this to thyroid suppression and progesterone deficiency, which furthern impair mitochondrial function. Eventually, energy bankruptcy leads to systemic collapse. Aside from stress hormones, Peat also attributed one’s depressed metabolic rate to PUFA oxidation and estrogen dominance.
Adaptations occur because of stress and are inherently taxing.
Maintaining the Resistance stage requires a constant energy loss. Prolonged elevation of stress hormones (e.g., cortisol) during Resistance has systemic effects that are necessary short-term, but detrimental long-term. As a result, the immune system is often suppressed during chronic stress adaptation, the brain alters neural pathways, and these changes can persist even after the stressor is removed.
It is because of this taxing adaptation that Peat and the bioenergetic community limit stressors like fasting (including intermittent), caloric restriction, low carb diets, intense cold water training, and over-exercising.
You may have heard the term “hormesis” used in the alt health community. Hormesis is a dose-response phenomenon where exposure to low doses of a stressor (chemical, physical, or biological) induces adaptive effects, while higher doses cause harm. This comes from the mindset "what doesn’t kill you makes you stronger," and claims that mild stressors can enhance resilience. Hormesis is the reason people tout practices like caloric restriction, intermittent fasting, cold water training, and phytochemical dosing of plant toxins like sulfurophane from broccoli.
With the background of Seyle’s GAS, Bioenergetics argues hormesis forces the body into the Resistance Stage, diverting energy from repair (e.g., fertility, muscle growth, cognitive function, gut health, detoxification) for survival. Hormesis advocates even depict the Hormetic Zone as the same location and state as Seyle’s Resistance Stage.
Stressors like fasting and cold exposure (via adrenaline and noradrenaline spikes) suppress thyroid hormones (T3/T4), reducing basal metabolic rate and mitochondrial efficiency to conserve energy but impairing long-term vitality. Bioenergetics prioritizes energy abundance (e.g., glucose metabolism) over stress "challenges." Hormesis increases cortisol and estrogen (linked to fibrosis, cancer) and decreases progesterone and thyroid function (critical for regeneration).
Hormesis is sometimes co-opted by industries to justify low-dose exposure to toxins (e.g., pesticides, radiation) under the guise of “beneficial stress.” The adaptation mechanism costs energy, it doesn’t create it. Low doses of toxins are simply lower toxicity than higher doses.
Exercise is sometimes explained as hormetic in nature when it is actually the direct effects of exercise - use of glucose, insulin sensitization, hypertrophy, and movement of lymph - that are the benefits, not the stress hormone release. Cortisol is released during exercise with the amount is determined by the intensity and duration relative to your threshold of tolerance.
Interestingly, the way anabolic steroids work is by dampening the cortisol response so that it isn't catabolic to muscle tissue. They’re not inherently anabolic, they’re anti-catabolic. This allows the body's natural anabolic processes (like protein synthesis triggered by exercise) to proceed more effectively. The most natural, easily available, anti-catabolic substance is sugar.
The important factor here is your personal Stress Tolerance Threshold…Overexercising is relative to your threshold, which is determined by our formula outlined earlier.
But your current environment isn’t the only thing impacting your personal Stress Tolerance Threshold.
The metabolic rate of the mother, determined by the balance of thyroid status to stress hormones, which in turn is determined by environmental factors (epigenetic) during the mother's life and the status of her mother, is passed to the fetus. This plays a significant role in fetal metabolic programming evidenced by the Dutch Hunger Winter study.
High maternal glucocorticoids (e.g., cortisol) reduce placental transfer of thyroid hormones and alter fetal hypothalamic-pituitary-thyroid (HPT) axis development. Prenatal stress correlates with reduced hippocampal volume and altered brain metabolites (e.g., choline, creatine) in offspring, impacting neurodevelopment.
The same thing is true for fathers - metabolic rate can be transferred through sperm.
Optimization of maternal thyroid function before and during pregnancy—particularly in the first trimester—is essential for ensuring healthy metabolically fit children. The first trimester establishes the child’s thyroid blueprint, dictating lifelong metabolic and cognitive health. NDT can help with optimizing thyroid status along with selenium (deiodinase support), enough calories, enough carbohydrates and progesterone.
Methylation is a biological process where a methyl group (CH₃) is added to DNA. This process is carried out by enzymes called DNA methyltransferases (DNMTs). It acts like a "switch," regulating gene activity by turning genes on or off without changing the DNA sequence itself.
Single Nucleotide Polymorphisms (SNPs) are variations in a single nucleotide (A, T, C, or G) at a specific position in the genome. For example, one person might have an "A" at a particular spot while another has a "G". These variations are common and can influence traits like disease susceptibility, drug response, or physical characteristics. SNPs affect methylation patterns.
Methylation modifies gene activity without altering the DNA sequence. SNPs are genetic variations that can influence traits and interact with processes like methylation.
When an organism experiences stress, it initially responds by altering methylation patterns. This methylation change is a rapid, reversible adaptation mechanism. Increased reliance on methylation serves as a buffer against environmental stressors. It allows for quick adjustments in gene expression without permanent genetic changes.
However, if the stress persists across generations SNPs may emerge as a more permanent adaptive response. SNPs represent genetic variations that can be passed down to offspring. This process forms a continuum of adaptation: from immediate methylation changes to long-term genetic variations (SNPs). It reflects the organism's attempt to find optimal responses to a poor environment over time.
Methylation changes (epigenetic) and SNPs (genetic) are not isolated but interact in the adaptive process.
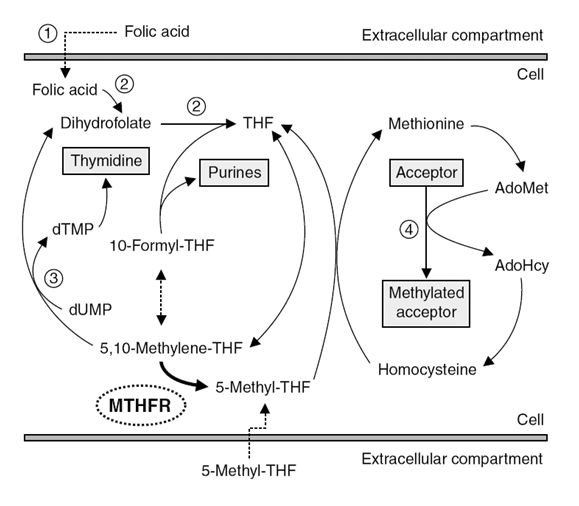
The MTHFR C677T polymorphism (rs1801133) is one of the most common and impactful SNPs globally, particularly in southern European, Hispanic and northern Chinese populations which can lower methylation by 35-90%. It’s central to folate metabolism and methylation, processes critical for DNA synthesis, neurotransmitter production, and stress response. SNPs like C677T (rs1801133) and A1298C (rs1801131) reduce MTHFR enzyme activity by ~35–70% (heterozygous) or ~70–90% (homozygous). Under stress, methylation demand spikes to regulate gene expression (e.g., stress-response genes like NR3C1 for cortisol receptors). Reduced MTHFR activity limits 5-methyl-THF (active folate), impairing SAMe production (the primary methyl donor).
This creates a methylation bottleneck, forcing prioritization of critical pathways (e.g., DNA repair over neurotransmitter synthesis). The short-term benefit is slower MTHFR activity may conserve folate for DNA synthesis during acute stress (e.g., famine), favoring survival. The long-term cost is that chronic stress exacerbates homocysteine (discussed last week) accumulation due to impaired conversion to methionine, increasing oxidative stress and cardiovascular/neuropsychiatric risks. SNPs like C677T may have optimized survival in low-folate, high-physical-stress environments (e.g., agrarian societies with seasonal folate-restricted diets). SNP carriers show higher depression risk after childhood trauma, linking MTHFR to stress sensitization.
If you ever had your genome mapped by a company like 23andMe you can download the raw data and import it into a site like Genetic Genie to identify if you have MTHFR SNPs (and any other SNPs). The standard method of remediating lowered folate conversion is to supplement with methyl-folate and methylated B12. If you have these SNPs its imperative that you avoid synthetic Folic Acid since the SNP significantly lowers conversion to methyl-folate. The buildup of unconverted Folic Acid can cause homocysteine accumulation and many other negative downstream consequences. Folic Acid is added to “fortified foods” and common in multivitamins, including prenatal.
Considering the trans-generational effects of metabolic heath, our new stress tolerance equation is:
[A + B = Metabolic rate] - [Current and Historical Net Stressors (physical and emotional)] = Your Current Stress Tolerance Threshold
A = Your Historical and Current Environment of Abundance/Scarcity
B = Trans-generational Parental Metabolic Health
Your personal Stress Tolerance Threshold is essentially an output of your environmental state of abundance/scarcity.
The good news is that you can significantly improve your metabolic status by following bioenergetic principles.
Key Takeaway:
Do your best to create an environment of abundance both physically and emotionally
To your health,
Jonathan
This is for informational purposes only and should not replace professional medical advice. Consult with your physician or other health care professional if you have any concerns or questions about your health.